System and Method for Biopsy Processing
A few months ago the Regenerative Medicine team reached a major milestone where we shipped our first system to a customer. Kevin was instrumental in the success of this project and was responsible for much of the mechanical design of this complex electro-mechanical system. The beta system has been installed for several months now requiring only minimal mechanical support, largely due to the design effort and collaboration spearheaded by Kevin. Getting the system to a point where it was ready to ship to the customer was not a trivial task and Kevin showed his commitment and character by working many long weeks and late nights, all while maintaining his focus and composure. Through the design, build, and testing of this system, Kevin worked cross functionally with other engineering disciplines and biologists to ensure the design functioned to specification.
Matthew White, Engineering Project Manager
Excerpt from Letter of Recommendation
The Mission
As part of DEKA Research & Development’s Regenerative Medicine team, we partnered with a client who had developed a cutting-edge autologous cell therapy to combat the limited treatment options for patients with chronic kidney disease (CKD), particularly those at end-stage. This therapy, leveraging a patient’s own cells to preserve kidney function, offered a potential alternative to dialysis, addressing a critical unmet need in CKD treatment.
However, the manual, time-intensive nature of the process restricted its scalability. Our team was tasked with automating key production steps, designing advanced equipment that would not only streamline operations but also enable wider access to this life-saving therapy. As part of their growth plan, the client intended to build a manufacturing plant equipped with our automated systems, ensuring efficient, large-scale production. Our automated system addressed the client’s need for scalability by replacing their manual, time-consuming process with a streamlined, efficient solution. By transforming their operations, we helped make the therapy more efficient, scalable, and accessible, ultimately broadening access to this therapy and increasing the potential to save lives.
The process involved extracting a small tissue sample from the patient's kidney through a standard biopsy. Through their process, the tissue is sent to a specialized facility, where the kidney cells are isolated, expanded, and selected for their therapeutic potential. These cells are then formulated into a personalized therapy and injected back into the patient’s kidneys to potentially restore function. This approach leverages the patient's own cells, reducing the need for immunosuppressive treatments and aiming to delay or prevent the progression to dialysis.
Project Responsibilities
System Design
Spearheaded the design and development of the automated biopsy processing system, driving the mechanical architecture and integration of key components. Worked closely with cross-functional teams in biology, systems, controls, and test engineering to understand user needs and optimize system performance.
Project Management
Leveraged strong project management skills to oversee the design, development, and delivery of complex systems. Managed multiple phases of the project, including setting timelines, coordinating cross-functional teams, and aligning project goals with client requirements. Effectively organized resources, minimized delays, and drove on-time project completion, all while balancing technical requirements with business objectives to ensure successful outcomes.
Detailed Drawings & Release
Produced and released over 200 detailed technical drawings for part fabrication across multiple assemblies and concept iterations, utilizing a range of manufacturing methods, including machining, sheet metal, injection molding, 3D printing, rapid prototyping, weldments, and ultrasonic and thermal welding. Implemented GD&T, tolerance analysis, and DfX principles to optimize part quality and fit across designs.
Process Validation
Applied process validation expertise to ensure the reliability of ultrasonic and thermal welding techniques used in disposable product assembly. Developed comprehensive validation protocols, verifying the integrity of welded joints and the sterility of components post-assembly. Authored detailed manufacturing instructions that incorporated best practices for disposable product assembly and sterilization.
V&V Testing
V&V testing included heater plate performance to ensure uniform and rapid heating, leak testing of fluid paths to maintain sterility, and validating tissue breakdown efficiency. Verified the integrity of disposable components under operational conditions, ensuring the system met both functional and regulatory standards. Evaluated cell yield to confirm optimal tissue digestion.
Vendor Relationships
Played a critical role in maintaining strong vendor relationships, working closely with our internal machine shop, external injection molding houses, and manufacturing teams to ensure smooth production workflows. Managed the delivery and installation of a prototype system at the client facility and remained on-site to troubleshoot and provide support as needed.
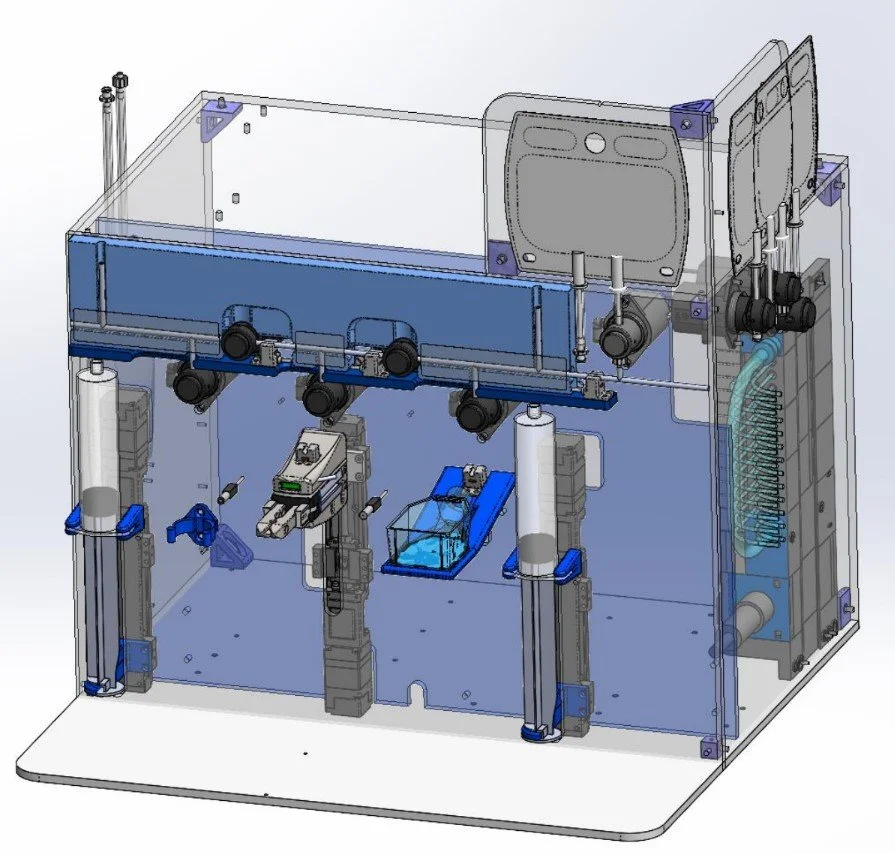
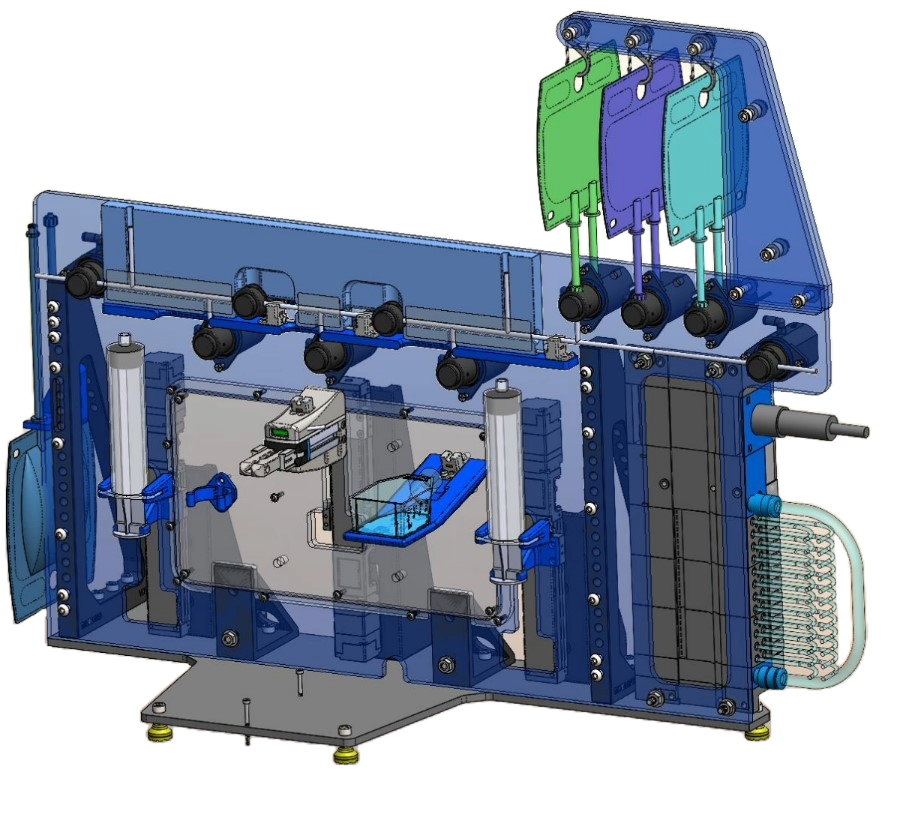
System Design Overview
Closed System Design
We developed a fully closed system to process biopsies, ensuring sterility throughout. The biopsy vial and tubing set were housed in a sterile environment, isolating the sample from external contaminants. This design enabled precise, automated control of fluid handling and tissue processing while maintaining a controlled environment.
Automated Biopsy Processing
The system utilized programmable logic controllers (PLCs) to synchronize various mechanical components, such as aspiration tips, linear grippers, orbital shakers, and heated plates. These components allowed for automated biopsy washing, digestion, and cell transfer, optimizing the entire process.
HMI
The Human-Machine Interface (HMI) provided an intuitive platform for users, guiding them through the process and allowing easy monitoring and control.
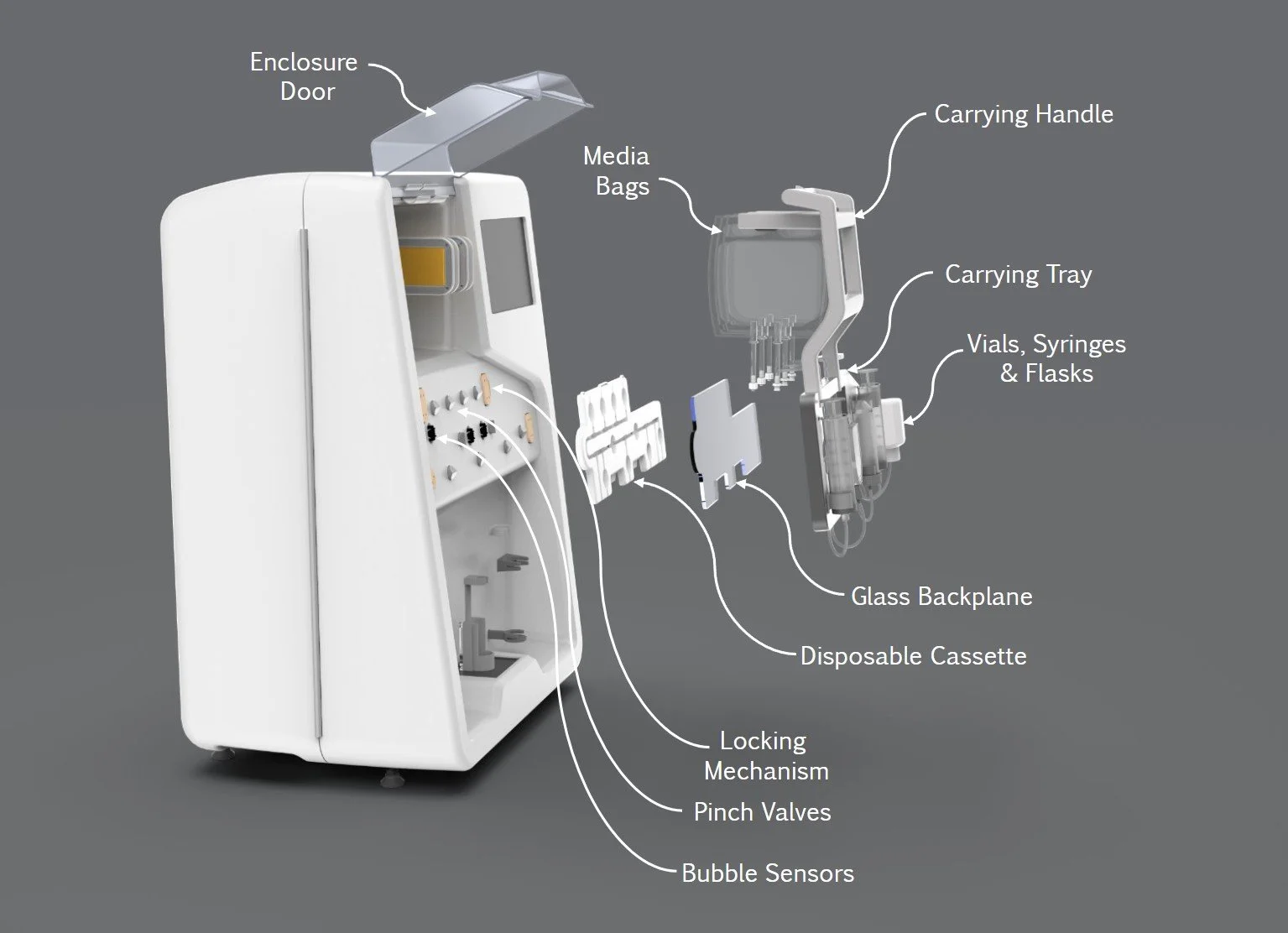
Cassette and Tubing System
The custom-designed disposable cassette and tubing set facilitated easy set-up and installation, while ergonomic carrying handles improved usability. The tubing set enabled sterile fluid transfers between the biopsy vial, wash/digest solutions, and final culture flasks. Once processed, the system automatically transferred the digested cells into a final culture flask, ready for further growth.
Mechanical Components
Aspiration & Gripper: A linear-actuated gripper controlled a pipette-like aspiration tip to manage fluid movement within the vial.
Shaker & Heater Plate: Orbital shakers and pneumatically-actuated heater plates were used to achieve mechanical agitation and precise thermal control.
Fluid Control: Pinch valves and syringe pumps regulated the flow of solutions, ensuring precise media exchanges and tissue digestion.
Carrying Tray & Disposable Cassette
The Challenge
System Requirements
Design Solution
The client’s autologous cell therapy process was highly labor intensive and costly. Additionally, the client sought to avoid further complexities to their clinical trials, and asked us to incorporate existing laboratory equipment that had been used to validate their process, including the vials, flasks, and media bags. They required an innovative solution to streamline their workflow from set up to completion, enabling scalability and cost-effectiveness without compromising the integrity of their established process.
Ease of Setup: Allow operators to quickly and accurately install disposable components with minimal effort and risk of errors.
Sterility: Maintain a closed, sterile system throughout the process to prevent contamination.
Scalability: Support high-throughput operations and manufacturing-scale processes with robust, modular design.
Cost Efficiency: Use materials and manufacturing methods that keep the components affordable while maintaining quality.
We developed an innovative cassette-based solution to streamline the organization and handling of vials, flasks, and media bags, transforming a labor intensive process into an ergonomic and efficient workflow. A pre-sterilized tubing set, including the cassette, vials, flasks, and syringes, arrived fully assembled for easy setup. Users would simply unpack and clip the cassette into a durable carrying tray, which also served as an assembly aid for sterile welding and connecting media bags. The carrying tray, equipped with a glass backstop for system pinch valves and a handle for ergonomic transport, facilitated seamless movement between setup stations and system installation.
The tray featured alignment mechanisms for precise system integration, locking securely in place during operation. Pinch valves controlled fluid flow in conjunction with the syringe pumps, while heater plates engaged with media bags to maintain process conditions. Vials, flasks, and syringes were transferred from the tray to system-specific holders, ensuring optimal functionality. During teardown, specific vials could be sterilely welded off, and the tray-cassette assembly was removed. The disposable cassette simplified waste management, while the reusable tray provided a sustainable solution for subsequent runs, enabling scalability, cost-effectiveness, and improved user experience.
Heater Plates
The Challenge
System Requirements
Design Solution
The enzymatic media used for tissue processing was temperature-sensitive, requiring elevated temperatures to be effective, yet was stored under refrigerated conditions. To optimize process efficiency and reduce overall processing time, we needed a solution that could rapidly bring the media to the desired temperature while maintaining precise control to ensure optimal conditions were achieved consistently.
Optimal Process Conditions: Ensure that enzymatic digestion and other critical phases occur at precise temperatures to maintain efficiency and cell viability.
Uniform Temperature Control: Avoid temperature gradients in fluids and samples to ensure consistency across batches.
Sterility: Maintain closed-system integrity to protect against contamination during heating.
Monitoring and Feedback: Include sensors to provide real-time temperature data to the programmable logic controller (PLC).
Safety: Overheat protection and redundant sensors to prevent sample, user, or equipment damage.
Pneumatically activated heater plates were designed and developed to achieve rapid and uniform heating of the media bags, ensuring consistent process conditions and minimizing temperature rise times. The pneumatic mechanism maintained constant compression on the media bags, allowing effective heating even as media volume decreased during the process. Silicone heating pads adhered to the heater plates provided efficient thermal transfer, while integrated RTDs, thermal cut-off (TCO) switches, and PLC control enabled precise temperature monitoring and regulation.
To ensure operational safety, the heater plates were housed within a thermally isolated compartment, minimizing heat dissipation and protecting system components. Hall effect sensors were incorporated for presence detection, ensuring the heaters were powered and the plates engaged only when the compartment door was securely closed. This design provided a robust and user-safe solution for maintaining optimal media temperatures throughout the process. Additionally, the media bags, mounted on the carrying handle, automatically aligned between the heater plates when the carrying tray was installed on the system, streamlining setup and improving usability. This design combined safety, efficiency, and ease of use to support consistent process performance.
Biopsy Vial Holder
The Challenge
System Requirements
Design Solution
The tissue sample was surgically extracted from the patient and stored in a standard vial and required sterile welding to the main tubing set during system setup. Throughout system operation, several process steps necessitated the user to sterilely seal and disconnect the sample vial for offline centrifugation, followed by reattachment to the system. To address these requirements, lab technicians needed a reliable method for easily and consistently inserting, aligning, and connecting the sample vial.
Additionally, the enzymatic tissue breakdown process required integrated mechanical agitation and heat to enhance the efficiency of tissue processing. The assembly also needed to incorporate multiple seals to safeguard the electronics during system cleaning and in the event of a leak, ensuring operational safety and durability. This design had to balance precision, ease of use, and robust protection in a demanding laboratory environment.
Secure Mounting: Ensure the sample vial is securely held in place during the process to avoid spillage or misalignment.
Thermal Control: Provide uniform heating or cooling to maintain the required temperature for enzymatic digestion or other process steps.
Agitation: Support orbital or mechanical agitation to assist tissue breakdown without damaging the vial or its contents.
Ease of Use: Allow operators to insert and remove vials quickly and accurately.
Sterility Maintenance: Minimize contamination risks by enabling closed-loop fluid handling and ensuring that the vial remains isolated from external environments.
Durability: Handle mechanical stresses such as agitation and fluid exchanges during high-throughput operations.
Our team designed a custom cap that attached to the sample vial using its existing threads and contained features that helped align the vial to a custom holder. This cap included an aspiration tip, similar to a pipette, enabling precise fluid control through a linear actuated gripper. The cap featured an integrated bellows to maintain sterility and a micron pore sized, thermally welded filter for venting. Additionally, the cap also contained features to assist in managing the tubing pigtail during centrifugation. The injection molded cap comprised of two halves ultrasonically welded together to capture the bellows. To maintain intellectual property confidentiality, only a high level description of the cap design is provided and no images are available.
Additionally, we used an off-the-shelf orbital shaker with a custom vial holder and spring-loaded RTD to ensure proper agitation and temperature monitoring. A protective custom bellows shielded electronics without interfering with shaker motion, ensuring process reliability.
Electronics Enclosure
The Challenge
System Requirements
Design Solution
Our system employed programmable logic controllers (PLCs) to coordinate and synchronize various components, including linear actuators, pinch valves, shakers, heaters, and sensors, for seamless process control. To ensure optimal functionality, the system needed to efficiently organize all PLC components and wiring, while also prioritizing accessibility for ease of service and troubleshooting.
Organization: The system should feature a well-organized layout for all PLC wiring to ensure operational reliability.
Serviceability and Troubleshooting: The system must provide easy access to the wiring and components for straightforward serviceability and troubleshooting without disrupting normal operations.
Heat and Vibration Management: Components, particularly the wiring and PLCs, should be thermally insulated and vibration-resistant to avoid damage during system operation.
We designed a sheet metal panel to securely mount all electronics, which was installed into an enclosure supported by a rigid welded frame to bear the weight of the heavy components. The system featured a swinging door configuration for easy access to internal mechanisms and electronics, ensuring serviceability. Cable management was achieved through dedicated ducts and service loops, connecting the front mechanisms to the back electronics. Louvers were molded into the cast urethane housing, with a refrigerator-style gasket seal preventing fluid ingress during cleaning, safeguarding the electronics.
Custom Injection Molded Caps
This high-level description omits specific design details to maintain intellectual property confidentiality.
To integrate standard vials and flasks into our tubing set solution, we designed custom injection-molded caps tailored for each component. These caps utilized the existing threads of the vials and flasks. Each cap featured a barb connection to securely and sterilely connect to our main tubing set. To enhance sealing performance, we incorporated lip seals to seal between the interface between the caps and their mating components. Additionally, micron pore sized filters were thermally welded to each cap to facilitate sterile venting during fluid delivery and prevent bacterial ingress. I led the design and manufacturing process for these caps. The manufacturing processes included optimizing ultrasonic and thermal welding parameters to ensure reliable assembly of components and conducting rigorous leak testing to validate sealing integrity and performance.
Agile Concept Development
We iterated often during early concept development stages in order to quickly gather end-user feedback and enhance the effectiveness & efficiency of our process development.
Concept 0.0
-
Laser cut acrylic panels and 3D printed parts (SLS & MJF Nylon). Total design and build time: 1 week.
-
Obtain end-user feedback on the proposed layout, ergonomics, and order of operations of the system. Understand assembly and serviceability of electrical and pneumatic wiring.
Concept 1.0
-
Water-jet, quarter-inch stainless steel plates for robustness. Design, part procurement, and assembly: 2 weeks.
-
Iterate on feedback from Concept 0.0. Optimize cassette design to improve disposable tubing, vial, and media bag management. First engineering unit developed to kickstart controls programming and process development.
Concept 2.0
-
Anodized, welded aluminum sheet metal enclosures. Design, fabrication, and assembly: 2 months
-
Refine cassette design with improved carrying ergonomics and loading onto system. Implement safety features such as enclosure door on heated plates, guard on orbital shaker, and eliminate pinch points on linear actuators.